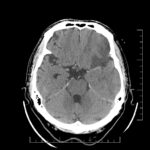
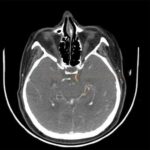
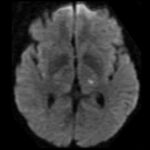
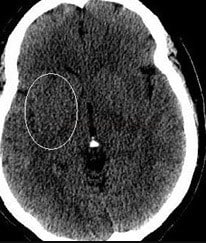
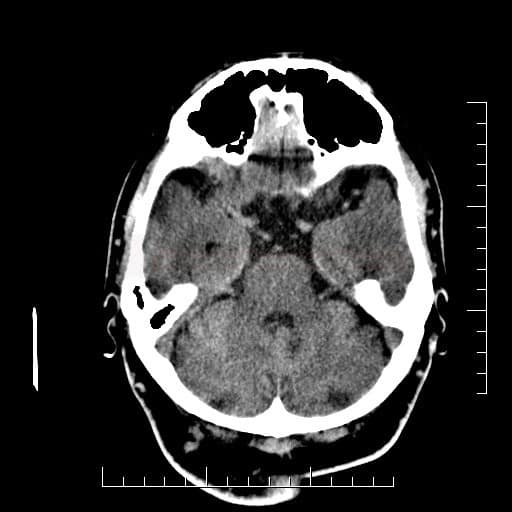
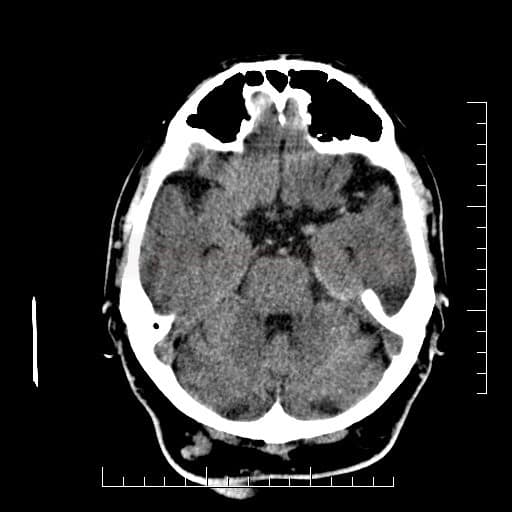
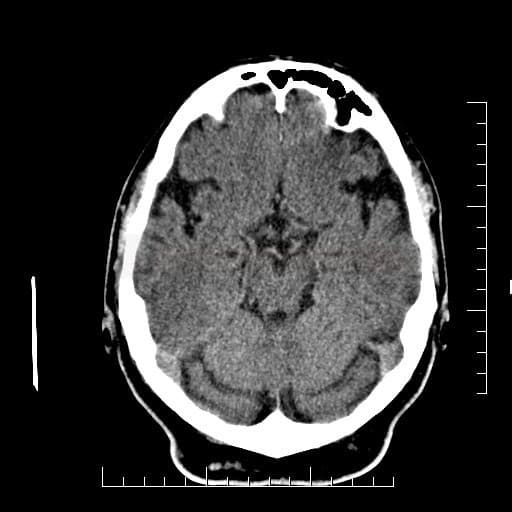
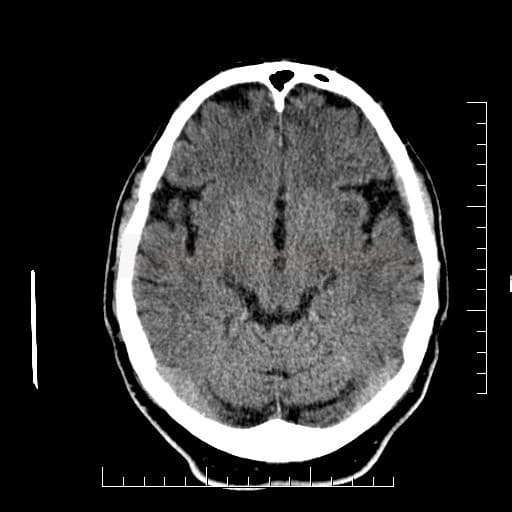
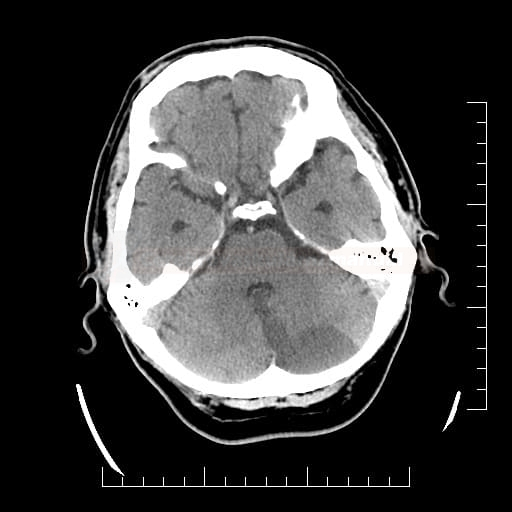
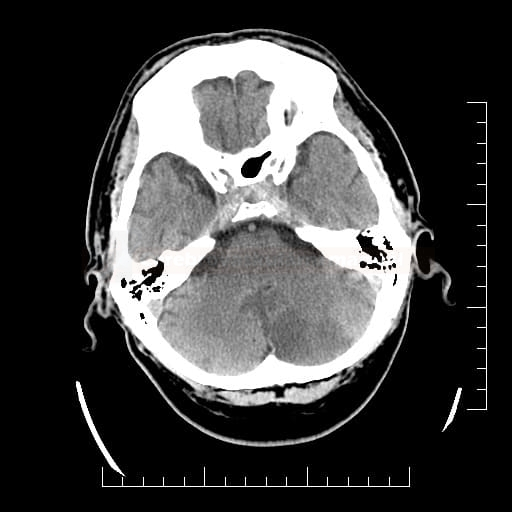
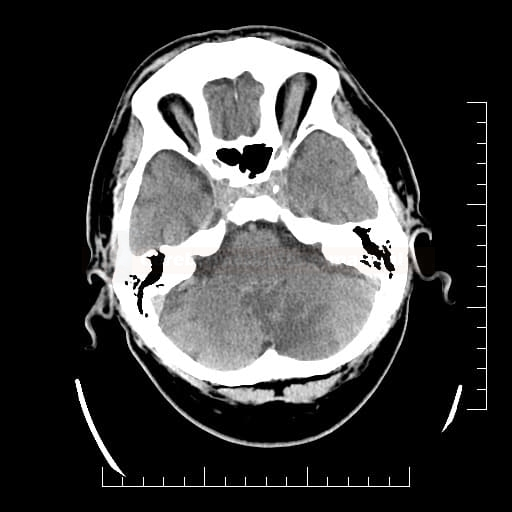
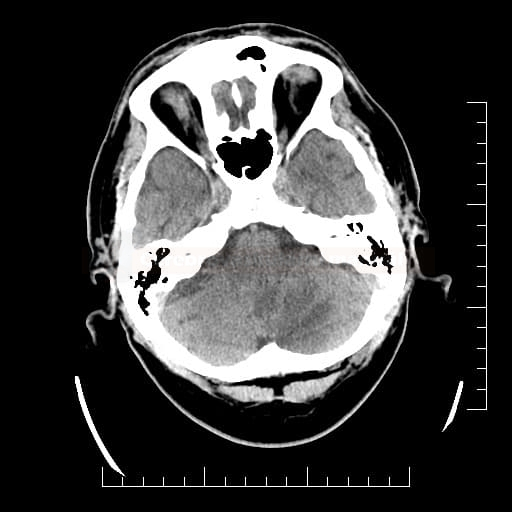
Cerebral Microbleeds
[toc]
Definition
- cerebral microbleeds (CMBs) or cerebral microhemorrhages are characterized by hemosiderin deposits caused by small hemorrhages and may serve as a radiologic biomarker of small vessel disease (SVD)
- black lesions on blood-sensitive MRI sequences (GRE T2* or SWI images)
- often found incidentally; the prevalence increases with age
- general population ∼ 10-15% (Sveinbjornsdottir, 2008)
- 6.5% of the individuals aged 45-50 years
- up to 40% of the population > 80 years [Poels, 2009]
- incidence of CMBs in AD is 20-43%; in vascular dementia, it is up to 85%! [Seo, 2007]
- microhemorrhages are associated with:
- older age (prevalence increasing significantly after the age of 75)
- hypertension
- smoking
- white matter disease and lacunar stroke
- previous ischemic stroke or intracerebral hemorrhage (ICH)
- COVID-19 leukoencephalopathy (mostly in critically ill patients) (Agarwal, 2020)
- a high number of microbleeds is associated with an increased risk of:
- cognitive impairment, which may progress to dementia (Werring, 2004) [Akoudad, 2006]
- intracerebral hemorrhage (especially with antithrombotic or fibrinolytic therapy)
- increased risk of progression is common in:
- severe SVD (Subcortical Vascular Disease / Small Vessel Disease)
- Cerebral amyloid angiopathy (CAA) with APOE ε2 and ε4 [McCarron, 2000]
Cerebral microbleeds and the risk of hemorrhage
- the risk of ICH increases with the number of CMBs
- ≥ 10 CMBs – OR for ICH 5.5!
- according to the CROMIS-2 trial, the risk of bleeding in patients with CMBs is 9.8/1000 vs. 2.6/1000 patient-years (adjusted hazard ratio 3·67, 95% CI 1·27–10·60) [Wilson, 2018]
- the risk of ICH is up to 5%/year in cases with multiple lobar CMBs [Van Etten, 2014]
- ≥ 5 CMBs = OR for ICH 2.8
| gfg | gfg |
black round or ovoid lesion with blooming on GRE/SWI |
g |
|
black round or ovoid lesion with blooming on GRE/SWI devoid of signal hyperintensity on T1- or T2-weighted sequences at least half surrounded by brain parenchyma distinct from other potential mimics such as iron/calcium deposits, bone, or vessel flow voids clinical history, excluding traumatic diffuse axonal injury (DAI) |
ggggg |
at least half surrounded by brain parenchyma |
g |
| gf |
distinct from other potential mimics such as iron/calcium deposits, bone, or vessel flow voids clinical history, excluding traumatic diffuse axonal injury (DAI) |
|
gggg |
Classification
- subcortical (mainly caused by arteriolopathy) → Binswanger’s disease
- cortical (mostly caused by CAA – with an increased risk of lobar hemorrhage)
- combined (combination of CAA and arteriolosclerosis or rather arteriolosclerosis alone) [Jung, 2020]
Radiographic features of hypertensive angiopathy
- cerebral microbleeds (predominantly in the deep grey nuclei and brainstem)
- subcortical infarcts (lacunar) in the deep grey nuclei, white matter, and brainstem
- dilated perivascular spaces in the basal ganglia
- white matter hyperintensities and hyperintensities in the deep grey nuclei and brainstem on T2
Test
- cerebral microbleeds (predominantly in the deep grey nuclei and brainstem)
Diagnostic evaluation
- detectable only on specific sequences, such as gradient-recalled echo (GRE) and susceptibility-weighted imaging (SWI)
- microbleeds are inapparent on other MRI sequences and CT
- black, round, or oval lesions measuring 2-5 mm in diameter, associated with a blooming artifact, which overestimates the size of the lesions
Greenberg’s criteria (Roob, 1999)
- black round or ovoid lesion with blooming on GRE/SWI
- devoid of signal hyperintensity on T1- or T2-weighted sequences
- at least half surrounded by brain parenchyma
- distinct from other potential mimics such as iron/calcium deposits, bone, or vessel flow voids
- clinical history, excluding traumatic diffuse axonal injury (DAI)
Radiographic features of hypertensive angiopathy
- both techniques are used to detect blood products and calcifications due to their sensitivity to local susceptibility effects
- T2*-weighted gradient-echo (GRE) sequences operate in 2D multi-slice mode, using relatively long TR’s, low flip angles, and relatively long TE’s
- modern susceptibility-weighted imaging (SWI) methods are based on GRE sequences but include numerous enhancements for improved differentiation between paramagnetic (hemorrhage) and diamagnetic (calcification) substances
- SWI is superior to GRE (particularly in the diagnosis of traumatic brain injury and microvascular angiopathy)
- however, SWI sequences take longer than standard GRE and are more susceptible to motion artifacts
Radiographic features of hypertensive angiopathy
It is a “susceptibility artifact” caused by paramagnetic substances
- hemosiderin
- cavernous malformation

- old hemorrhages, microbleeds

- SAH (even small superficial)
- diffuse axonal injury (DAI)

- superficial siderosis
- older thrombus
- detection of cerebral venous thrombosis (CVT)

- excessive blooming from hemosiderin is an unfavorable predictor of recanalization
 [Chen, 2015]
[Chen, 2015]
- detection of cerebral venous thrombosis (CVT)
- cavernous malformation
- calcification
- e.g., neurocysticercosis
- Fahr disease

- metals
- gas
- due to the artifact, SWI is highly sensitive in detecting even small lesions, particularly those associated with hemorrhage or mineralization
Differential Diagnosis
- calcium and iron deposits (calcium is hyperdense on the CT scan)

- diseases with an accumulation of iron → see here
- flow void from veins or small arteries on the cross-section

- follow the continuum of the vessel on adjacent slices
- cavernous malformation (cavernoma)

- malignant melanoma metastases

- T1 – hyperintense (due to bleeding and/or the presence of melanin)
- T2 – hypointense
- T1 C+ – diffuse or ring-like saturation
- T2*- hypointense
- pneumocephalus and gas embolism
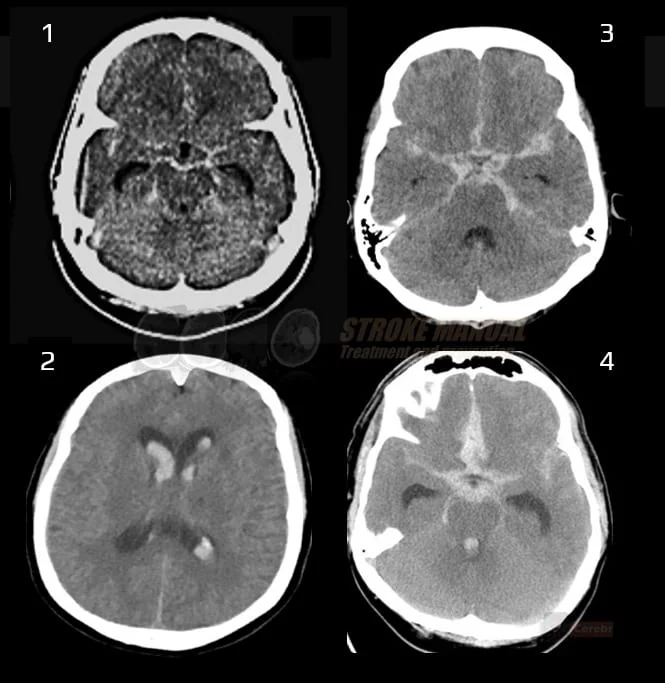
- metallic emboli from mechanical heart valve (very rare)
Management
subscription is required to see this content
- the risk of symptomatic intracranial hemorrhage (sICH) may increase after thrombolytic therapy in patients with cerebral microbleeds (CMBs)
- CMBs < 10 ⇒ IVT seems safe (AHA/ASA 2019 IIa/B-NR)
- CMBs > 10 ⇒ IVT carries a higher risk of ICH; the expected benefit of treatment must outweigh the risk ⇒ consider IVT in patients with a severe stroke
- a small study retrospectively found a slightly increased risk (3%) of bleeding in patients with microbleeds on GRE [Fiehler, 2007]
- an increased risk of bleeding is associated with CAA as a cause of microbleeds
- MRI screening is not recommended to assess CMB burden before making a treatment decision regarding IVT
test text
- no definitive guidelines exist for antiplatelet use in patients with CMBs
- single antiplatelet therapy – seems a safe and beneficial approach (RESTART trial subanalysis) [Salman, 2019]
- dual antiplatelet therapy (DAPT) – individual risk-benefit analysis is crucial (DAPT is acceptable after recent stenting, etc.)
Radiographic features of hypertensive angiopathy
- cerebral microbleeds (predominantly in the deep grey nuclei and brainstem)
- subcortical infarcts (lacunar) in the deep grey nuclei, white matter, and brainstem
- dilated perivascular spaces in the basal ganglia
- white matter hyperintensities and hyperintensities in the deep grey nuclei and brainstem on T2
Test
- cerebral microbleeds (predominantly in the deep grey nuclei and brainstem)
Test2
- the risk of symptomatic intracranial hemorrhage (sICH) may increase after thrombolytic therapy in patients with cerebral microbleeds (CMBs)
- CMBs < 10 ⇒ IVT seems safe (AHA/ASA 2019 IIa/B-NR)
- CMBs > 10 ⇒ IVT carries a higher risk of ICH; the expected benefit of treatment must outweigh the risk ⇒ consider IVT in patients with a severe stroke
- a small study retrospectively found a slightly increased risk (3%) of bleeding in patients with microbleeds on GRE [Fiehler, 2007]
- an increased risk of bleeding is associated with CAA as a cause of microbleeds
- MRI screening is not recommended to assess CMB burden before making a treatment decision regarding IVT
test text
- no definitive guidelines exist for antiplatelet use in patients with CMBs
- single antiplatelet therapy – seems a safe and beneficial approach (RESTART trial subanalysis) [Salman, 2019]
- dual antiplatelet therapy (DAPT) – individual risk-benefit analysis is crucial (DAPT is acceptable after recent stenting, etc.)